Steady-state voltammetry at a microdisc electrode in the absence of excess supporting electrolyte for reversible, quasi-reversible and irreversible electrode kinetics
Abstract
The steady-state 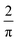 the radius) is shown to be valid under weakly supported conditions, for a range of electrochemical rate constants
the radius) is shown to be valid under weakly supported conditions, for a range of electrochemical rate constants 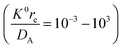 . The simulations were used, in conjunction with the Debye–Hückel theory, to rationalise the experimental steady-state
. The simulations were used, in conjunction with the Debye–Hückel theory, to rationalise the experimental steady-state
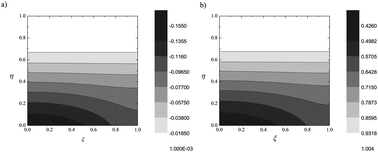

 Please wait while we load your content...
Please wait while we load your content...