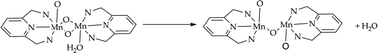
By using first-principles molecular dynamics simulations combined with metadynamics to simulate rare events we analyse competing reaction coordinates for a di-Mn water oxidation catalyst ([(bis(imino)pyridine)(H2O)MnIV(μ-O)2MnV(O)(bis(imino)pyridine)]3+). The catalytic water oxidation cycle of the complex is examined by addressing the thermodynamic accessibility of the hydroperoxo species that is considered a critical and rate-limiting intermediate. To achieve this, hybrid quantum-mechanics/molecular-mechanics (QM/MM) and full QM simulations have been performed for an explicit treatment of the water environment that plays an active role in the reaction processes. Starting from a likely active species for the O–O bond formation, we observe that during the water approach to the oxo ligand a facile structural rearrangement of the complex takes place, leading to the opening of one μ-O bridge and the release of a water ligand, and resulting in two pentacoordinated Mn centers. This complex appears weakly active in the water oxidation process, since a concerted reaction is required to establish a Mn–OOH hydroperoxo intermediate. The slow kinetics of a concerted reaction can allow other processes, including linear degradation of the catalyst, to take precedence over catalytic water oxidation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...