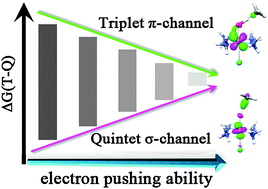
Comprehensive density functional theory computations on substrate hydroxylation by a range of nonheme iron(IV)–oxo model systems [FeIV(O)(NH3)4L]+ (where L = CF3CO2−, F−, Cl−, N3−, NCS−, NC−, OH−) have been investigated to establish the effects of axial ligands with different degrees of electron donor ability on the reactivity of the distinct reaction channels. The results show that the electron-pushing capability of the axial ligand can exert a considerable influence on the different reaction channels. The σ-pathway reactivity decreases as the electron-donating ability of the axial ligand strengthens, while the π-pathway reactivity follows an opposite trend. Moreover, the apparently antielectrophilic trend observed for the energy gap between the triplet π- and quintet σ-channel (ΔG(T–Q)) stems from the fact that the reaction reactivity can be fine-controlled by the interplay between the exchange-stabilization benefiting from the 5TSH relative to the 3TSH by most nonheme enzymes and the destabilization effect of the 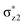 orbital by the anionic axial ligand. When the former counteracts the latter, the quintet σ-pathway will be more effective than the other alternatives. Nevertheless, when the dramatic destabilization effect of the
orbital by the anionic axial ligand. When the former counteracts the latter, the quintet σ-pathway will be more effective than the other alternatives. Nevertheless, when the dramatic destabilization effect of the 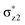 orbital by a strong binding axial σ-donor ligand like OH− counteracts but does not override the exchange-stabilization, the barrier in the quintet σ-pathway will remain identical to the triplet π-pathway barrier. Indeed, the axial ligand does not change the intrinsic reaction mechanism in its respective pathway; however, it can affect the energy barriers of different reaction channels for C–H activation. As such, the tuning of the reactivity of the different reaction channels can be realised by increasing/decreasing the electron pushing ability.
orbital by a strong binding axial σ-donor ligand like OH− counteracts but does not override the exchange-stabilization, the barrier in the quintet σ-pathway will remain identical to the triplet π-pathway barrier. Indeed, the axial ligand does not change the intrinsic reaction mechanism in its respective pathway; however, it can affect the energy barriers of different reaction channels for C–H activation. As such, the tuning of the reactivity of the different reaction channels can be realised by increasing/decreasing the electron pushing ability.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
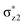 orbital by the anionic axial
orbital by the anionic axial 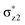 orbital by a strong binding axial σ-donor
orbital by a strong binding axial σ-donor 

 Please wait while we load your content...
Please wait while we load your content...