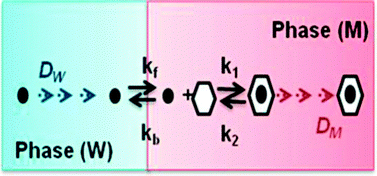
General analytical equations corresponding to the Facilitated Ion Transfer (FIT) at ITIES (Interface between Two Immiscible Electrolyte Solutions) are presented for the most frequent case in which the complexing agent is present only in the organic phase, and considering both the ion transfer and the chemical complexation kinetic effects. Under these conditions, the FIT process can be regarded as an EC mechanism. This study is of great interest to elucidate the origin of the kinetic effects which affect the electrochemical signal. Normal Pulse Voltammetry and Derivative Normal Pulse Voltammetry are chosen as representative and easy understandable voltammetric techniques. From the general equations, the expressions corresponding to some interesting particular situations in which one or the two kinetic processes (ion transfer and organic complexation) are in equilibrium are derived. Moreover, working curves of the characteristic peak parameters of the derivative voltammetric response are given, from which it is possible to determine the kinetic constants. The results obtained here are applicable to a wide range of liquid membrane systems, from traditional liquid–liquid interfaces to plasticized polymeric membranes and supported liquid–liquid interfaces.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...