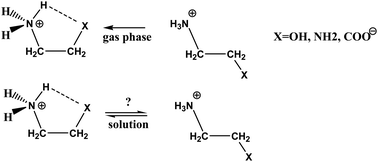
The gauche–trans conformational equilibrium has been studied for +H3NCH2CH2X systems (X = OH, NH2, COO−) and for the neutral model of the simplest β-amino acid in aqueous solution and chloroform. Each structure exhibits an intramolecular hydrogen bond in the NCCX gauche conformation, which is necessarily disrupted in the local energy minimum trans form. Geometries were optimized at the IEF-PCM/B97D/aug-cc-pvtz level of theory and indicated that the solvent effect of chloroform vs. water is moderate when the geometries for the corresponding gauche and trans conformers are compared. The only remarkable difference was found for β-alanine, which can exist in gauche, zwitterionic form in aqueous solution but not in chloroform. Total relative free energies were obtained as the sum of the relative IEF-PCM/B97D/aug-cc-pvtz internal free energy and ΔG(solv). The latter was calculated both by means of IEF-PCM and the explicit solvent FEP/Monte Carlo methods. The resulting ΔGtot values could differ by 1–2 kcal mol−1 depending on the accepted ΔG(solv) value, but any calculation indicated that the internally bound gauche conformers are far more populated than the corresponding trans species. A difference of 2.6 kcal mol−1 for ΔG(solv) by the two methods resulted in the preference of the zwitterionic gauche β-alanine structure by FEP/MC in contrast to IEF-PCM favoring the neutral form in aqueous solution. Monte Carlo characterization of the solution structure in the first solvation shell of polar groups indicates that solvation of a N–H+ bond is sensitive to its involvement in an intramolecular hydrogen bond. For the zwitterionic, gauche β-alanine, almost no water oxygen can reach that N–H+ bond, which is in strong interaction with the –COO− group by forming a six-membered ring and favorable local geometry for the hydrogen bond.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...