The role of protein hydrophobicity in thionin–phospholipid interactions: a comparison of α1 and α2-purothionin adsorbed anionic phospholipid monolayers†
Abstract
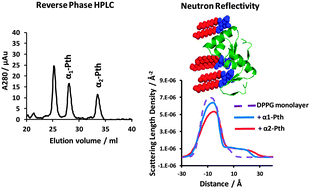
The plant defence proteins α1- and α2-purothionin (Pth) are type 1 thionins from common wheat (Triticum aestivum). These highly homologous proteins possess characteristics common amongst antimicrobial peptides and proteins, that is, cationic charge, amphiphilicity and hydrophobicity. Both α1- and α2-Pth possess the same net charge, but differ in relative hydrophobicity as determined by C18 reversed phase HPLC. Brewster angle microscopy, X-ray and neutron reflectometry, external reflection FTIR and associated surface pressure measurements demonstrated that α1 and α2-Pth interact strongly with condensed phase 1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DPPG) monolayers at the air/liquid interface. Both thionins disrupted the in-plane structure of the anionic phospholipid monolayers, removing lipid during this process and both penetrated the lipid monolayer in addition to adsorbing as a single protein layer to the lipid head-group. However, analysis of the interfacial structures revealed that the α2-Pth showed faster disruption of the lipid film and removed more phospholipid (12%) from the interface than α1-Pth. Correlating the protein properties and lipid binding activity suggests that hydrophobicity plays a key role in the membrane lipid removal activity of thionins.

 Please wait while we load your content...
Please wait while we load your content...