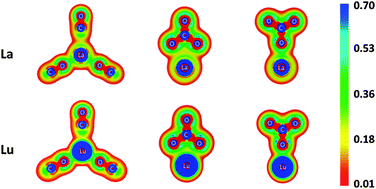
Quantum chemical calculations were employed to elucidate the structural and bonding properties of La(III) and Lu(III) carbonates. These elements are found at the beginning and end of the lanthanoid series, respectively, and we investigate two possible metal-carbonate stoichiometries (tri- and tetracarbonates) considering all possible carbonate binding motifs, i.e., combinations of mono- and bidentate coordination. In the gas phase, the most stable tricarbonate complexes coordinate all carbonates in a bidentate fashion, while the most stable tetracarbonate complexes incorporate entirely monodentate carbonate ligands. When continuum aqueous solvation effects are included, structures having fully bidentate coordination are the most favorable in each instance. Investigation of the electronic structures of these species reveals the metal–ligand interactions to be essentially devoid of covalent character.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...