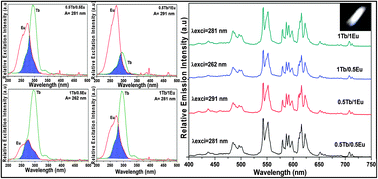
Trivalent rare-earth (RE) ions (Eu3+, Tb3+ and Sm3+) activated multicolor emitting SrY2O4 phosphors were synthesized by a sol–gel process. The structural and morphological studies were performed by the measurements of X-ray diffraction profiles and scanning electron microscope (SEM) images. The pure phase of SrY2O4 appeared after annealing at 1300 °C and the doping of RE ions did not show any effect on the structural properties. From the SEM images, the closely packed particles were observed due to the roughness of each particle tip. The photoluminescence (PL) analysis of individual RE ions activated SrY2O4 phosphors exhibits excellent emission properties in their respective regions. The Eu3+ co-activated SrY2O4:Tb3+ phosphor creates different emissions by controlling the energy transfer from Tb3+ to Eu3+ ions. Based on the excitation wavelengths, multiple (green, orange and white) emissions were obtained by Sm3+ ions co-activated with SrY2O4:Tb3+ phosphors. The decay measurements were carried out for analyzing the energy transfer efficiency and the possible ways of energy transfer from donor to acceptor. The cathodoluminescence properties of these phosphors show similar behavior as PL properties except the energy transfer process. The obtained results indicated that the energy transfer process was quite opposite to the PL properties. The calculated CIE chromaticity coordinates of RE ions activated SrY2O4 phosphors confirmed the red, green, orange and white emissions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...