Model oxide-supported metal catalysts – comparison of ultrahigh vacuum and solution based preparation of Pd nanoparticles on a single-crystalline oxide substrate
Abstract
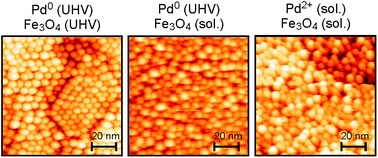
Using single-crystalline Fe3O4(111) films grown over Pt(111) in UHV as a model-support, we have characterized the nucleation behaviour and chemical properties of Pd particles grown over the film using different deposition techniques with

 Please wait while we load your content...
Please wait while we load your content...