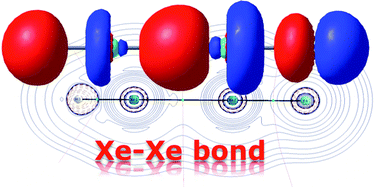
The structure and stability towards decomposition of eight novel noble gas compounds having a Xe–Xe bond, which have not been experimentally observed so far, have been studied computationally. In addition, the nature of the Xe–Xe interaction has been analysed by a combination of the most popular methods to study the bonding situation of molecules, i.e. Natural Bond Orbital, Atom in Molecules and Energy Decomposition Analysis methods. Two related series of compounds have been considered: HXeXeX (X = F to I) and RXeXeR′ (R = halogen atom). Our calculations indicate that the replacement of the fluorine atom by a heavier group 17 congener in the HXeXeX series leads to a less stable compound, thus making more difficult its experimental observation. The same effect occurs in the RXeXeR′ series, but these species are more kinetically protected against the decomposition reaction and therefore, their experimental detection is more likely.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...