The inhibitory effects of vinylphosphonate-linked thymidine dimers on the unidirectional translocation of PcrA helicase along DNA: a molecular modelling study†
Abstract
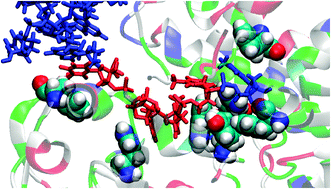
The PcrA DNA helicases are important bacterial enzymes and quintessential examples of molecular motors. Through conformational changes caused by ATP

 Please wait while we load your content...
Please wait while we load your content...