Arsenic in prebiotic species: a theoretical approach
Abstract
A recent controversy about the presence of arsenic in biological systems prompted us to investigate the possible replacement of phosphorus by arsenic in prebiotic species small enough to be potentially identified in space. Systematic computational experiments were carried out on simple systems able to form a ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) is not the most stable structure, contrary to NH2–CH
O) is not the most stable structure, contrary to NH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. It is ∼9 kcal mol−1 higher than the most stable structure, CH2
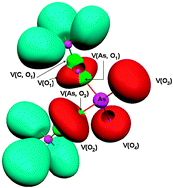
O. It is ∼9 kcal mol−1 higher than the most stable structure, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) As–OH. To assess the plausibility of the As to P substitution, a comparative study of the dimethylphosphate (DMP) and dimethylarsenate (DMA) anions was then carried out. It was found that the gauche–gauche arrangement that mimics the helix structure is the most stable one in both model molecules, showing that there is no structural evidence to discard the hypothesis of the possible inclusion of As in place of P in the DNA architecture. The topological analysis of the ELF function showed a weakening by 50% of two As–O covalent bonds in all the DMA conformers. It means that if As replaces P, the structure of the DNA helix could be weakened. Rotational constants and
As–OH. To assess the plausibility of the As to P substitution, a comparative study of the dimethylphosphate (DMP) and dimethylarsenate (DMA) anions was then carried out. It was found that the gauche–gauche arrangement that mimics the helix structure is the most stable one in both model molecules, showing that there is no structural evidence to discard the hypothesis of the possible inclusion of As in place of P in the DNA architecture. The topological analysis of the ELF function showed a weakening by 50% of two As–O covalent bonds in all the DMA conformers. It means that if As replaces P, the structure of the DNA helix could be weakened. Rotational constants and

 Please wait while we load your content...
Please wait while we load your content...