Modeling the influence of adsorbed DNA on the lateral pressure and tilt transition of a zwitterionic lipid monolayer
Abstract
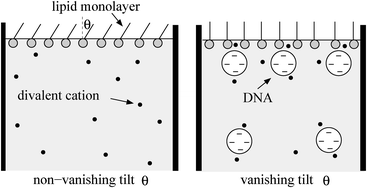
Certain lipid monolayers at the air–water interface undergo a second-order transition from a tilted to an untilted liquid-crystalline state of their lipid hydrocarbon chains at sufficiently large lateral pressure. Recent experimental observations demonstrate that in the presence of divalent cations DNA adsorbs onto a zwitterionic lipid monolayer and decreases the tilt transition pressure. Lowering of the tilt transition pressure indicates that the DNA condenses the lipid monolayer laterally. To rationalize this finding we analyze a theoretical model that combines a phenomenological Landau approach with an extension of the Poisson–Boltzmann model to zwitterionic lipids. Based on numerical calculations of the mean-field electrostatic free energy of a zwitterionic lipid monolayer–DNA complex in the presence of divalent cations, we analyze the thermodynamic equilibrium of DNA adsorption. We find that adsorbed DNA induces a 10% reduction of the electrostatic contribution to the lateral pressure exerted by the monolayer. This result implies a small but notable decrease in the tilt transition pressure. Additional mechanisms due to ion–ion correlations and headgroup reorientations are likely to further enhance this decrease.

 Please wait while we load your content...
Please wait while we load your content...