Controlling the rate of electron transfer between a quantum dot and a tri-ruthenium molecular cluster by tuning the chemistry of the interface†
Abstract
Ultrafast transient absorption measurements reveal that the rate of photoinduced
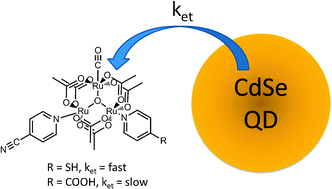
- This article is part of the themed collection: Electron Transfer Theory

 Please wait while we load your content...
Please wait while we load your content...