Light induced water oxidation on cobalt-phosphate (Co–Pi) catalyst modified semi-transparent, porous SiO2–BiVO4 electrodes†
Abstract
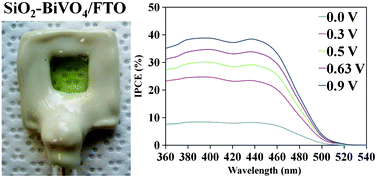
A facile and simple procedure for the synthesis of semi-transparent and porous SiO2–BiVO4 electrodes is reported. The method involves a

 Please wait while we load your content...
Please wait while we load your content...