Solubility of gold nanoparticles as a function of ligand shell and alkane solvent
Abstract
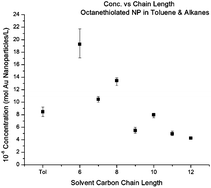
The solubility of ca. 5.0 nm gold nanoparticles was studied systematically as a function of ligand shell and solvent. The ligands were octane-, decane-, dodecane- and hexadecanethiols; the solvents were the n-alkanes from hexane to hexadecane and toluene. Supernatant concentrations in equilibrium with precipitated superclusters of nanoparticles were measured at room temperature (23 °C) with UV-Vis spectrophotometry. The solubility of nanoparticles ligated with decane- and dodecanethiol was greatest in n-decane and n-dodecane, respectively. In contrast, the solubility of nanoparticles ligated with octane- and hexadecanethiol showed decreasing solubility with increasing solvent chain length. In addition the solubility of the octanethiol ligated system showed a nonmonotonic solvent carbon number functionality with even numbered solvents being better solvents than neighboring odd numbered solvents.

 Please wait while we load your content...
Please wait while we load your content...