Experimental and theoretical study of the reaction of the ethynyl radical with nitrous oxide, C2H + N2O†
Abstract
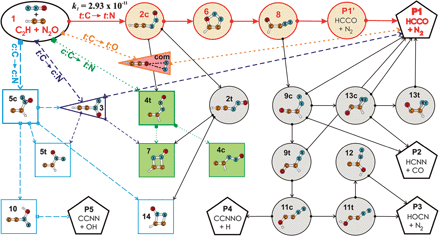
We investigated the rate constants and reaction mechanism of the gas phase reaction between the ethynyl radical and nitrous oxide (C2H + N2O) using both experimental methods and electronic structure calculations. A pulsed-laser photolysis/chemiluminescence technique was used to determine the absolute rate coefficient over the temperature range 570 K to 836 K. In this experimental temperature range, the measured temperature dependence of the overall rate constants can be expressed as: k(T) (C2H + N2O) = 2.93 × 10−11 exp((−4000 ± 1100) K/T) cm3 s−1 (95% statistical confidence). Portions of the C2H + N2O potential energy surface (PES), containing low-energy pathways, were constructed using the composite G3B3 method. A multi-step reaction route leading to the products HCCO + N2 is clearly preferred. The high selectivity between product channels favouring N2 formation occurs very early. The pathway corresponds to the addition of the terminal C atom of C2H to the terminal N atom of N2O. Refined calculations using the coupled-cluster theory whose electronic energies were extrapolated to the complete basis set limit CCSD(T)/CBS led to an energy barrier of 6.0 kcal mol−1 for the entrance channel. The overall rate constant was also determined by application of transition-state theory and Rice–Ramsperger–Kassel–Marcus (RRKM) statistical analyses to the PES. The computed rate constants have similar temperature dependence to the experimental values, though were somewhat lower.

 Please wait while we load your content...
Please wait while we load your content...