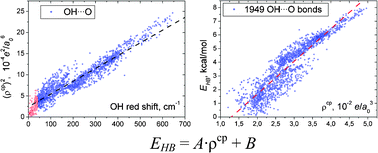
Physical properties of over 8000 intramolecular hydrogen bonds (iHBs), including 2901 ones of the types OH⋯O, OH⋯N, NH⋯O and OH⋯C, in 4244 conformers of the DNA-related molecules (four canonical 2′-deoxyribonucleotides, 1,2-dideoxyribose-5-phosphate, and 2-deoxy-D-ribose in its furanose, pyranose and linear forms) have been investigated using quantum theory of atoms in molecules (QTAIM) and vibrational analysis. It has been found that for all iHBs with positive red-shift of the proton donating group stretching frequency the shift value correlates with ρcp—the electron charge density at the (3,−1)-type bond critical point. Combining QTAIM and spectroscopic data new relationships for estimation of OH⋯O, OH⋯N, NH⋯O and OH⋯C iHB enthalpy of formation (kcal mol−1) with RMS error below 0.8 kcal mol−1 have been established: EOH⋯O = −3.09 + 239·ρcp, EOH⋯N = 1.72 + 142·ρcp, ENH⋯O = −2.03 + 225·ρcp, EOH⋯C = −0.29 + 288·ρcp, where ρcp is in e a0−3 (a0 – the Bohr radius). It has been shown that XH⋯Y iHBs with red-shift values over 40 cm−1 are characterized by the following minimal values of the XHY angle, ρcp and ∇2ρcp: 112°, 0.005 e a0−3 and 0.016 e a0−5, respectively. New relationships have been used to reveal the strongest iHBs in canonical 2′-deoxy- and ribonucleosides and the O5′H⋯N3 H-bond in ribonucleoside guanosine was found to have the maximum energy (8.1 kcal mol−1).

 Please wait while we load your content...
Please wait while we load your content...