Tuning the band gaps and work functions via topology and carbon concentration: a first-principles investigation of Cx(BN)y compounds†
Abstract
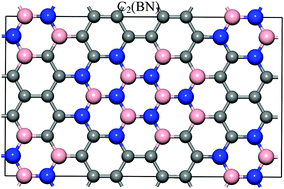
The electronic properties, stabilities, and work functions of Cx(BN)y monolayers were systematically investigated by first-principle techniques. The results indicated that the band gaps of the systems are rather sensitive to the topology and symmetry. However, the formation energies clearly suggested that the BN dimers tend to be grouped to one side and the carbon atoms are grouped to the other side. Such an atomic arrangement has the lowest formation energy and is thermodynamically highly stable, and furthermore their band gaps decrease gradually with an increasing of carbon content. Further analysis revealed that the band gap narrowing of G(I) structures depends on the nature of the C-2pz and N-2pz states. In contrast to the electronic properties, the variation of work functions as functions of carbon content exhibits an opposite trend. The strong correlation between the positive charge (Qpos.tot.) : work function (WCx(BN)y) ratio and carbon content indicated that the ionicity of Cx(BN)y compounds can be controlled by the carbon content and therefore determine the work functions of the systems.

 Please wait while we load your content...
Please wait while we load your content...