Excited-state dynamics and efficient triplet formation in phenylthiophene compounds
Abstract
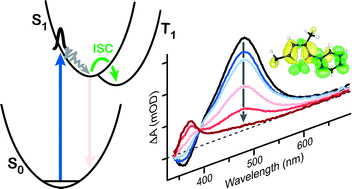
Ultrafast transient absorption spectroscopy monitors the solution-phase dynamics of 2-phenylthiophene (PT), 2-methyl-5-phenylthiophene (MPT), and 2,4-dimethyl-5-phenylthiophene (DMPT) following excitation to the first singlet excited state. Rapid spectral evolution indicates that structural relaxation on the S1 potential energy surface occurs within ∼100 fs, whereas the picosecond-scale kinetics reveal efficient intersystem crossing to the triplet manifold of states. The rate of intersystem crossing is significantly faster for DMPT (21.6 ± 1.0 ps) than for PT (102 ± 5 ps) and MPT (132 ± 3 ps). The measurements provide new limits on the timescale for a competing isomerization reaction in which the phenyl group changes position on the thiophene ring. The role of methyl substitution in driving the intersystem crossing is discussed.
- This article is part of the themed collection: Ultrafast chemical dynamics

 Please wait while we load your content...
Please wait while we load your content...