Carbon monoxide adsorption on neutral and cationic vanadium doped gold clusters†
Abstract
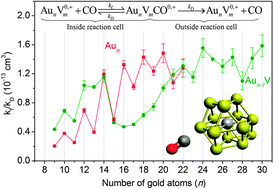
The effect of a single vanadium dopant atom on the reactivity of small gold clusters is studied in the gas phase. In particular we investigated carbon monoxide
- This article is part of the themed collection: Structure and reactivity of small particles: from clusters to aerosols

 Please wait while we load your content...
Please wait while we load your content...