Unraveling the Fischer–Tropsch mechanism: a combined DFT and microkinetic investigation of C–C bond formation on Ru
Abstract
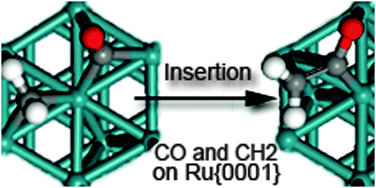
A combined modelling study on the Fischer–Tropsch Mechanism on Ru(0001). The DFT results presented herein approve the idea that the carbide mechanism is not the main reaction path in the synthesis of liquid

 Please wait while we load your content...
Please wait while we load your content...