Redox and photoisomerization switching of the second-order optical nonlinearity of a tetrathiafulvalene derivative of spiropyran across five states: a DFT study†
Abstract
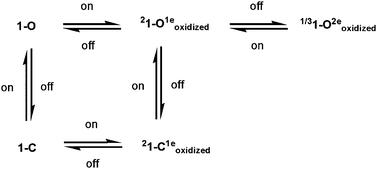
Second-order nonlinear optical properties of a tetrathiafulvalene (TTF) derivative of spiropyran have been studied based on density functional theory (DFT) combined with the finite field (FF) calculations. Our DFT-FF calculations confirm a switching behavior of the static first hyperpolarizability caused by the redox and photochromic reaction. The photochromic reaction generates spiropyran–merocyanine conversion by reversible cleavage of the C–O bond, which is relative to the close- and open-ring forms 1-c and 1-o. The open-ring form 1-o displays the large static first hyperpolarizability relative to its close-ring form 1-c according to our DFT-FF calculations with three functionals. The electronic structure analysis and spin unrestricted calculations show that the redox processes significantly affect the geometrical structure of the TTF unit, and thus enhance the static first hyperpolarizabilities. The one-electron-oxidized species having good planar structure of the TTF unit are ∼30 and ∼200 times as large as that of the neutral compounds 1-c and 1-o, respectively. But the difference in the static first hyperpolarizability between one- and two-electron-oxidized states of spiropyran species is not substantial according to our DFT-FF calculations, and the spiropyran–merocyanine conversion of two-electron-oxidized species does not largely affect their static first hyperpolarizability. On the basis of the large change in the static first hyperpolarizability, our DFT-FF calculations support a five-state switching of the static first hyperpolarizability based on the redox and photoisomerization.

 Please wait while we load your content...
Please wait while we load your content...