3D-RISM-KH molecular theory of solvation and density functional theory investigation of the role of water in the aggregation of model asphaltenes†
Abstract
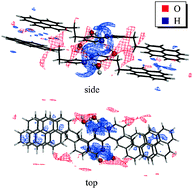
We applied a multiscale modeling approach that involves the statistical–mechanical three-dimensional reference interaction site model with the Kovalenko–Hirata closure approximation (3D-RISM-KH molecular theory of solvation) as well as density functional theory (DFT) of electronic structure to study the role of

 Please wait while we load your content...
Please wait while we load your content...