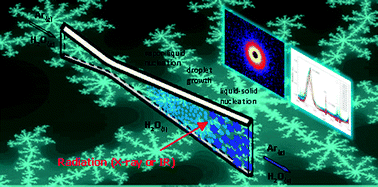
We report homogeneous ice nucleation rates between 202 K and 215 K, thereby reducing the measurement gap that previously existed between 203 K and 228 K. These temperatures are significantly below the homogenous freezing limit, TH ≈ 235 K for bulk water, and well within no-man's land. The ice nucleation rates are determined by characterizing nanodroplets with radii between 3.2 and 5.8 nm produced in a supersonic nozzle using three techniques: (1) pressure trace measurements to determine the properties of the flow as well as the temperature and velocity of the droplets, (2) small angle X-ray scattering (SAXS) to measure the size and number density of the droplets, and (3) Fourier Transform Infrared (FTIR) spectroscopy to follow the liquid to solid phase transition. Assuming that nucleation occurs throughout the droplet volume, the measured ice nucleation rates Jice,V are on the order of 1023 cm−3 s−1, and agree well with published values near 203 K.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...