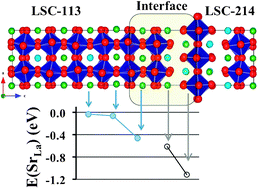
An interface between the perovskite La0.8Sr0.2CoO3−δ (LSC-113) and the K2NiF4-type (La0.5Sr0.5)2CoO4−δ (LSC-214) heterostructure was recently shown to enhance oxygen surface exchange and the rate of the oxygen reduction reaction (ORR) by orders of magnitude compared to either the LSC-113 or LSC-214 phase alone. This result is of interest to develop better optimized materials for solid-state electrochemical devices, e.g. solid oxide fuel cells. The effect has been attributed to the interface itself, rather than changes in the bulk LSC-113 or LSC-214 phases. Using density functional theory (DFT)-based simulations, we demonstrate that there is a ∼0.9 eV (∼1.3 eV) energy gain for exchanging a Sr from LSC-113(25%Sr) (LSC-113(40%Sr)) with a La from LSC-214(50%Sr). These changes in energy create a large driving force for interdiffusion across the heterostructure interface from Sr into LSC-214 and La into LSC-113. We estimate that the Sr concentrations (in the LSC-214 phase) in a typical experimental temperature range of 500–600 °C and in equilibrium with LSC-113(25%Sr) and LSC-113(40%Sr), may be about 75% Sr and 90% Sr, respectively. Based on the bulk behavior of the LSC-214 phase (Vashook et al., Solid State Ionics, 2000, 138, 99–104), an Sr enrichment from x = 0.5 to x = 0.75 in (La1−xSrx)2CoO4−δ is expected to enhance the oxygen vacancy concentration by 2–2.5 orders of magnitude under typical experimental conditions. An increased vacancy concentration in LSC-214 near the interface can explain most of the enhanced oxygen kinetics observed up until now in these heterostructures.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...