A theoretic insight into the catalytic activity promotion of CeO2 surfaces by Mn doping†
Abstract
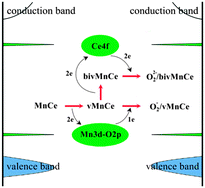
In this paper, we investigated the primary reduction and oxygen replenishing processes over Mn substitutionally doped CeO2(111) surfaces by density functional theory with the on-site Coulomb correction (DFT + U). The results indicated that Mn doping could make the surface much more reducible and the adsorbed O2 could be effectively activated to form superoxo (O2−) and/or peroxo species (O22−). The Mn doping induced the Mn 3d–O 2p gap state instead of Ce 4f acting as an electrons acceptor and donor during the first oxygen vacancy formation and O2 replenishing, which helped to lower the formation energy of the first and second oxygen vacancies to −0.46 eV and 1.40 eV, respectively. In contrast, the formation energy of a single oxygen vacancy in the pure ceria surface was 2.08 eV and only peroxo species were identified as the O2 molecule adsorbed. Our work provides a theoretical and electronic insight into the catalytic redox processes of Mn doped ceria surfaces, which may help to understand the enhanced catalytic performances of MnOx–CeO2 oxides, as reported in previous experimental works.

 Please wait while we load your content...
Please wait while we load your content...