Interaction of acetone, hydroxyacetone, acetaldehyde and benzaldehyde with the surface of water ice and HNO3·3H2O ice†
Abstract
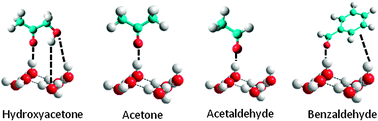
Oxygenated volatile organic compounds (OVOCs) influence the oxidative properties of the atmosphere, and their transport from the ground may occur by scavenging by the HNO3-rich supercooled

 Please wait while we load your content...
Please wait while we load your content...