Non-monotonic dependence of water reorientation dynamics on surface hydrophilicity: competing effects of the hydration structure and hydrogen-bond strength
Abstract
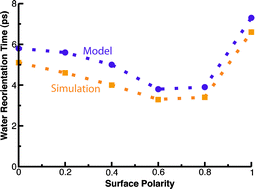
The reorientation dynamics of interfacial
- This article is part of the themed collection: Physics and chemistry of ice and water

 Please wait while we load your content...
Please wait while we load your content...