Surface models and reaction barrier in Eley–Rideal formation of H2 on graphitic surfaces
Abstract
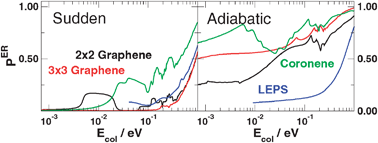
The exothermic, collinearly-dominated Eley–Rideal hydrogen formation on graphite is studied with electronic structure and quantum dynamical means. In particular, the focus is on the importance of the model used to describe the graphitic substrate, in light of the marked discrepancies present in available literature results. To this end, the collinear reaction is considered and the potential energy surface is computed for a number of different graphitic surface models using Density Functional Theory (DFT) for different dynamical regimes. Quantum dynamics is performed with wavepacket techniques down to the cold collision energies relevant for the chemistry of the interstellar medium. Results show that the reactivity at moderate-to-high collision energies sensitively depends on the shape of the PES in the entrance channel, which in turn is related to the adopted surface model. At low energies we rule out the presence of any barrier to reaction, thereby highlighting the importance of quantum reflection in limiting the reaction efficiency.

 Please wait while we load your content...
Please wait while we load your content...