Formation of Al2H7− anions — indirect evidence of volatile AlH3 on sodium alanate using solid-state NMR spectroscopy†‡
Abstract
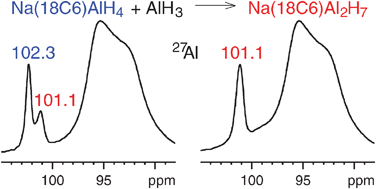
After more than a decade of intense research on NaAlH4 doped with transition metals as hydrogen storage material, the actual mechanism of the decomposition and rehydrogenation reaction is still unclear. Early on, monomeric AlH3 was named as a possible transport shuttle for aluminium, but never observed experimentally. Here we report for the first time the trapping of volatile AlH3 produced during the decomposition of undoped NaAlH4 by an adduct of sodium alanate and

 Please wait while we load your content...
Please wait while we load your content...