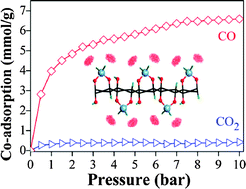
We propose titanium-decorated graphene oxide (Ti–GO) as an ideal sorbent for carbon monoxide (CO) capture and separation from gas mixtures. Based on first-principles calculations, Ti–GO exhibits a large binding energy of ∼70 kJ mol−1 for CO molecules, while the binding energies for other gases, such as N2, CO2, and CH4, are significantly smaller. The gas adsorption properties of Ti–GO are independent of the local GO structures once Ti atoms are anchored by the oxygen-containing groups on the GO surface. The strong interaction between CO molecule and Ti is a result of dative bonding, i.e., hybridization between an empty d orbital of Ti and an occupied p orbital of CO. Adsorption isotherms from grand canonical Monte Carlo simulations clearly demonstrate the strong selectivity of Ti–GO for CO adsorption in a mixture with other gas.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...