Investigating the CO2 uncaging mechanism of nitrophenylacetates by means of fs-IR spectroscopy and quantum chemical calculations
Abstract
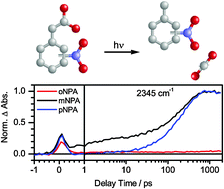
Caged compounds are widely utilized for light-triggered control of biological and chemical reactions. In our study we investigated the photo-induced decarboxylation of all three constitutional isomers of nitrophenylacetate (NPA), which can be regarded as caged-CO2. UV-pump/IR-probe

 Please wait while we load your content...
Please wait while we load your content...