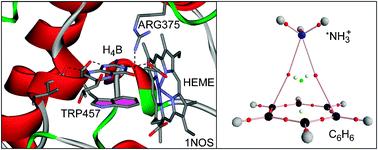
Cation and anion–π interactions are important binding forces where aromatic rings are involved. In this manuscript we define and compare two related interactions that have not been reported so far, namely radical cation and radical anion–π interactions (C˙+–π and A˙−–π, respectively). Moreover, we compare the energetic features of these complexes with the related closed-shell interactions. Interestingly the C˙+–π interaction is more favourable than the standard cation–π interaction and the contrary is observed for the A˙−–π interaction. Changes in the aromatic character of the ring upon complexation of the ion have been studied using the nucleus-independent chemical shift (NICS) criterion. Orbitalic and spin density calculations of the complexes have been computed in order to investigate if spin transfer effects between the radical ions and the aromatic ring exist. We have analyzed the physical nature of the interactions by means of Bader's theory of “atoms-in-molecules” and partitioning the total interaction energy into individual components using the Molecular Interaction Potential with a polarization partition scheme. Finally, we describe an interesting biological example, which involves the tetrahydrobiopterin cofactor, where the presence of a radical ion–π interaction is important.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...