Femtosecond broadband time-resolved fluorescence and transient absorption study of the intramolecular charge transfer state of methyl 4-dimethylaminobenzoate
Abstract
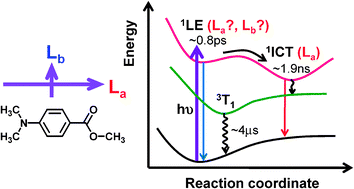
A combined application of femtosecond broadband time-resolved fluorescence (fs-TRF),

 Please wait while we load your content...
Please wait while we load your content...