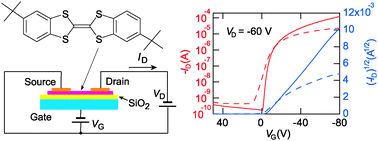
For a material for organic thin-film transistors, not only high mobility but also low threshold voltage and long-term stability are important requirements. In order to realize these properties, materials with relatively large oxidation potentials, namely weak donors, have been designed as p-channel organic semiconductors. Here we propose a different strategy; transistor properties of dibenzotetrathiafulvalene (DBTTF) are significantly improved by the introduction of tert-butyl groups. Although this chemical modification does not much change the ionization potential, small threshold voltage and stability over several months are attained together with the improved mobility, probably due to some kind of passivation effect of the bulky tert-butyl groups. In contrast, the systematic fluorine substitution rapidly diminishes the transistor performance. There are two kinds of herringbone structures with much different dihedral angles of about 50° and 130°, and the tert-butyl compound falls into the former category.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...