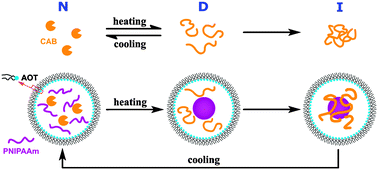
At high temperature, many enzymes are inactivated by aggregations at hydrophobic sites which are exposed on denaturation. Isolating denatured enzymes via hydrophobic interactions with other material is a significant method to prevent enzymes from aggregation. But the temperature-sensitive polymer poly(N-isopropylacrylamide) (PNIPAAm), supposed to protect enzymes spontaneously at high temperatures, can not efficiently complex denatured carbonic anhydrase B (CAB, as a model enzyme) in bulk aqueous solution due to different phase transition speeds. Here, we present a novel method for protecting enzymes against heat inactivation, in which PNIPAAm and CAB are encapsulated in a confined space constructed by reverse microemulsion. At high temperatures, PNIPAAm forms nanoscale aggregates possessing both large specific surface areas and hydrophobic surfaces, and then adsorbs denatured CABvia hydrophobic interactions to avoid intermolecular aggregation of CAB. With cooling, CAB is released spontaneously and recovers its activity. The assays for enzymatic activity demonstrate that CAB is effectively protected against heat inactivation through this method (protection efficiency is up to 83.2%).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...