Inelastic collisions of ultracold polar LiCs molecules with caesium atoms in an optical dipole trap
Abstract
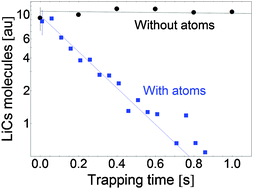
We investigate collisions of ultracold polar LiCs molecules and ultracold caesium atoms. LiCs molecules are formed in an optical dipole trap by photoassociation of caesium and lithium atoms via the B1Π excited state followed by spontaneous emission to the X1Σ+ground state and the lowest triplet state a3Σ+. The molecules are then stored together with caesium atoms in the same optical trap. Rate coefficients for the loss of molecules induced by collisions with surrounding Cs atoms are measured for molecular ensembles produced via different photoassociation resonances. The results are analyzed in terms of the unitarity limit for the inelastic rates and predictions from the universal model of Idziaszek and Julienne (Phys. Rev. Lett., 2010, 104, 113202).
- This article is part of the themed collection: Physics and chemistry of cold molecules

 Please wait while we load your content...
Please wait while we load your content...