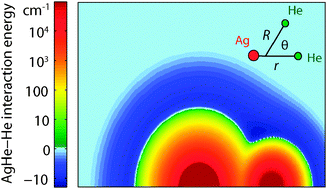
We show that weakly bound He-containing van der Waals molecules can be produced and magnetically trapped in buffer-gas cooling experiments, and provide a general model for the formation and dynamics of these molecules. Our analysis shows that, at typical experimental parameters, thermodynamics favors the formation of van der Waals complexes composed of a helium atom bound to most open-shell atoms and molecules, and that complex formation occurs quickly enough to ensure chemical equilibrium. For molecular pairs composed of a He atom and an S-state atom, the molecular spin is stable during formation, dissociation, and collisions, and thus these molecules can be magnetically trapped. Collisional spin relaxation is too slow to affect trap lifetimes. However, 3He-containing complexes can change spin due to adiabatic crossings between trapped and untrapped Zeeman states, mediated by the anisotropic hyperfine interaction, causing trap loss. We provide a detailed model for Ag3He molecules, using ab initio calculation of Ag–He interaction potentials and spin interactions, quantum scattering theory, and direct Monte Carlo simulations to describe formation and spin relaxation in this system. The calculated rate of spin-change agrees quantitatively with experimental observations, providing indirect evidence for molecular formation in buffer-gas-cooled magnetic traps. Finally, we discuss the possibilities for spectroscopic detection of these complexes, including a calculation of expected spectra for Ag3He, and report on our spectroscopic search for Ag3He, which produced a null result.

 Please wait while we load your content...
Please wait while we load your content...