He-atom scattering from MgO(100): calculating diffraction peak intensities with a semi ab initio potential
Abstract
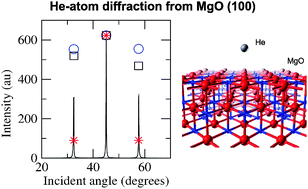
An efficient model describing the He-atom scattering process is presented. The He–surface interaction potential is calculated from first principles by exploiting second-order Rayleigh–Schrödinger many-body perturbation theory and fitted by using a variety of pairwise interaction potentials. The attractive part of the fitted analytical form has been upscaled to compensate the underestimation of the well depth for this system in the perturbation theory description. The improved potential has been introduced in the close-coupling method to calculate the diffraction pattern. Quantitative agreement between the computed and observed binding energy and diffraction intensities for the He–MgO(100) system is achieved. It is expected that the utility of He scattering for probing dynamical processes at surfaces will be significantly enhanced by this quantitative description.

 Please wait while we load your content...
Please wait while we load your content...