From a localized H3O radical to a delocalized H3O+⋯e−solvent-separated pair by sequential hydration†
Abstract
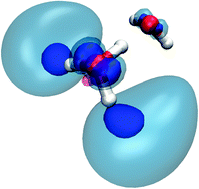
The impact of microhydration on the electronic structure and reactivity of the H3O moiety is investigated by ab initio calculations. In the gas phase, H3O is a radical with spin density localized on its hydrogen end, which is only kinetically stable and readily decomposes into a
- This article is part of the themed collection: Weak hydrogen bonds – strong effects?
 Please wait while we load your content...
Please wait while we load your content...