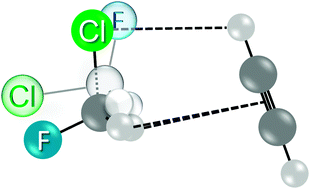
The structure of the CH2ClF⋯HCCH dimer has been determined using both chirped-pulse and resonant cavity Fourier-transform microwave spectroscopy. The complex has Cs symmetry and contains both a double C–H⋯π interaction, in which one π-bond acts as acceptor to two hydrogen atoms from the CH2ClF donor, and a weak C–H⋯Cl interaction, with acetylene as the donor. Analysis of the rotational spectra of four isotopologues (CH235ClF⋯H12C12CH, CH237ClF⋯H12C12CH, CH235ClF⋯H13C13CH, and CH237ClF–H13C13CH) has led to a structure with C–H⋯π distances of 3.236(6) Å and a C–H⋯Cl distance of 3.207(22) Å, in good agreement with ab initio calculations at the MP2/6-311++G(2d,2p) level. Both weak contacts are longer than those observed in similar complexes containing a single C–H⋯π interaction that lies in the Cs plane; however, this appears to be the first double C–H⋯π contact to be studied by microwave spectroscopy, so there is little data for direct comparison. The rotational and chlorine nuclear quadrupole coupling constants for the most abundant isotopologue are: A = 5262.899(14) MHz, B = 1546.8074(10) MHz, C = 1205.4349(7) MHz, χaa = 28.497(5) MHz, χbb = −65.618(13) MHz, and χcc = 37.121(8) MHz.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...