Existence of both blue-shifting hydrogen bond and Lewis acid–base interaction in the complexes of carbonyls and thiocarbonyls with carbon dioxide†
Abstract
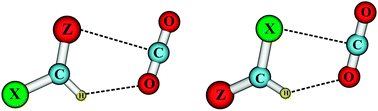
In this study, 16 gas phase complexes of the pairs of XCHZ and CO2 (X = F, Cl, Br; Z = O, S) have been identified. Interaction energies calculated at the CCSD(T)/aug-cc-pVTZ//MP2/aug-cc-pVTZ level including both BSSE and ZPE corrections range from −5.6 to −10.5 kJ mol−1 for XCHO⋯CO2 and from −5.7 to −9.1 kJ mol−1 for XCHS⋯CO2. Substitution of one H atom by one ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S3⋯C6 to >C1
S3⋯C6 to >C1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O3⋯C6 and to >C1–X4⋯C6. This result suggests the higher capacity of solubility of
O3⋯C6 and to >C1–X4⋯C6. This result suggests the higher capacity of solubility of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S functional group in competition with >C
S functional group in competition with >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The Lewis acid–base interaction of the types >C
O. The Lewis acid–base interaction of the types >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S⋯C, >C–Cl⋯C and >C–Br⋯C is demonstrated for the first time. The contribution of the hydrogen bonding interaction to the total interaction energy is larger for XCHS⋯CO2 than for XCHO⋯CO2. Upon complexation, a contraction of the C1–H2 bond length and a blue shift of its stretching frequency have been observed, as compared to the isolated monomer, indicating the existence of a blue-shifting hydrogen bond in all complexes examined. Calculated results also lend further support for the viewpoint that when acting as
S⋯C, >C–Cl⋯C and >C–Br⋯C is demonstrated for the first time. The contribution of the hydrogen bonding interaction to the total interaction energy is larger for XCHS⋯CO2 than for XCHO⋯CO2. Upon complexation, a contraction of the C1–H2 bond length and a blue shift of its stretching frequency have been observed, as compared to the isolated monomer, indicating the existence of a blue-shifting hydrogen bond in all complexes examined. Calculated results also lend further support for the viewpoint that when acting as
- This article is part of the themed collection: Weak hydrogen bonds – strong effects?
 Please wait while we load your content...
Please wait while we load your content...