Ion-transfer- and photo-electrochemistry at liquid|liquid|solid electrode triple phase boundary junctions: perspectives
Abstract
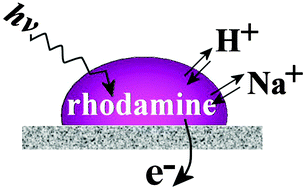
Ion transfer at liquid|liquid junctions is one of the most fundamental processes in nature. It occurs coupled to simultaneous

 Please wait while we load your content...
Please wait while we load your content...