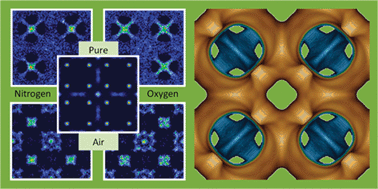
We have used interatomic potential-based simulations to study the removal of carbon tetrachloride from air at 298 K, using Cu–BTC metal organic framework. We have developed new sets of Lennard-Jones parameters that accurately describe the vapour–liquid equilibrium curves of carbon tetrachloride and the main components from air (oxygen, nitrogen, and argon). Using these parameters we performed Monte Carlo simulations for the following systems: (a) single component adsorption of carbon tetrachloride, oxygen, nitrogen, and argon molecules, (b) binary Ar/CCl4, O2/CCl4, and N2/CCl4 mixtures with bulk gas compositions 99 : 1 and 99.9 : 0.1, (c) ternary O2/N2/Ar mixtures with both, equimolar and 21 : 78 : 1 bulk gas composition, (d) quaternary mixture formed by 0.1% of CCl4 pollutant, 20.979% O2, 77.922% N2, and 0.999% Ar, and (e) five-component mixtures corresponding to 0.1% of CCl4 pollutant in air with relative humidity ranging from 0 to 100%. The carbon tetrachloride adsorption selectivity and the self-diffusivity and preferential sitting of the different molecules in the structure are studied for all the systems.

 Please wait while we load your content...
Please wait while we load your content...