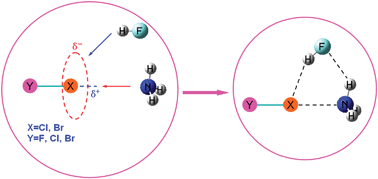
A series of ring-shaped molecular complexes formed by H3N, HF and XY (X = Cl, Br and Y = F, Cl, Br) have been investigated at the MP2/aug-cc-pVTZ level of theory. Their optimized geometry, stretching mode, and interaction energy have been obtained. We found that each complex possesses two red-shifted hydrogen bonds and one red-shifted halogen bond, and the two hydrogen bonds exhibit strong cooperative effects on the halogen bond. The cooperativity among the NH3⋯FH, FH⋯XY and H3N⋯XY interactions leads to the formations of these complexes. The AIM analysis has been performed at the CCSD(T)/aug-cc-pVQZ level of theory to examine the topological characteristics at the bond critical point and at the ring critical point, confirming the coexistence of the two hydrogen bonds and one halogen bond for each complex. The NBO analysis carried out at the B3LYP/aug-cc-pVTZ level of theory demonstrates the effects of hyperconjugation, hybridization, and polarization coming into play during the hydrogen and halogen bonding formations processes, based on which a clockwise loop of charge transfer was discovered. The molecular electrostatic potential has been employed to explore the formation mechanisms of these molecular complexes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...