A study of the ethene-ozone reaction with photoelectron spectroscopy: measurement of product branching ratios and atmospheric implications†
Abstract
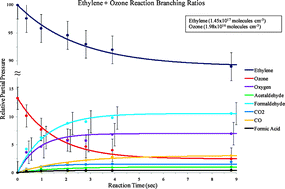
The ozone-ethene reaction has been investigated at low pressure in a flow-tube interfaced to a u.v. photoelectron

 Please wait while we load your content...
Please wait while we load your content...