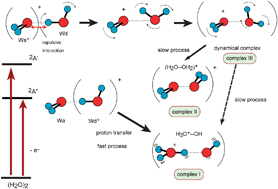
Ionization dynamics of a water dimer have been investigated by means of a direct ab initio molecular dynamics (MD) method. Two electronic state potential energy surfaces of (H2O)2+ (ground and first excited states, 2A′′ and 2A′) were examined as cationic states of (H2O)2+. Three intermediate complexes were found as product channels. One is a proton transfer channel where a proton of H2O+ is transferred into the H2O and then a complex composed of H3O+(OH) was formed. The second is a face-to-face complex channel denoted by (H2O–OH2)+ where the oxygen–oxygen atoms directly bind each other. Both water molecules are equivalent to each other. The third one is a dynamical complex where H2O+ and H2O interact weakly and vibrate largely with a large intermolecular amplitude motion. The dynamics calculations showed that in the ionization to the 2A′′ state, a proton transfer complex H3O+(OH) is only formed as a long-lived complex. On the other hand, in the ionization to the 2A′ state, two complexes, the face-to-face and dynamical complexes, were found as product channels. The proton of H2O+ was transferred to H2O within 25–50 fs at the 2A′′ state, meaning that the proton transfer on the ground state is a very fast process. On the other hand, the decay process on the first excited state is a slow process due to the molecular rotation. The mechanism of the ionization dynamics of (H2O)2 was discussed on the basis of theoretical results.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...