Reactive uptake kinetics of NO3 on multicomponent and multiphase organic mixtures containing unsaturated and saturated organics
Abstract
We investigated the reactive uptake of 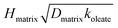 varied by a factor of 2.7, and assuming a surface reaction HSmatrixKSmatrixkSoleate varied by a factor of 3.6, where
varied by a factor of 2.7, and assuming a surface reaction HSmatrixKSmatrixkSoleate varied by a factor of 3.6, where 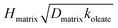 and HSmatrixKSmatrixkSoleate are constants extracted from the data using the resistor model. For solid–liquid mixtures, the reactive uptake coefficient depended on exposure time: the uptake decreased by a factor of 10 after exposure to
and HSmatrixKSmatrixkSoleate are constants extracted from the data using the resistor model. For solid–liquid mixtures, the reactive uptake coefficient depended on exposure time: the uptake decreased by a factor of 10 after exposure to


 Please wait while we load your content...
Please wait while we load your content...