Molecular beam studies of HCl dissolution and dissociation in cold salty water
Abstract
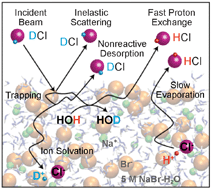
Gas-liquid scattering experiments are used to explore collisions and reactions of HCl and DCl with 12 mol% LiBr solutions of
- This article is part of the themed collection: Molecular Collision Dynamics

 Please wait while we load your content...
Please wait while we load your content...